3) First-Ever Genetic Mutation Discovered in CD
The first-ever genetic mutation was found in a Castleman disease patient. Bob Ohgami, MD, PhD, a CDCN-funded physician-scientist at Stanford, discovered that a somatic mutation in DNMT3A was found in lymph node tissue in 1 of 3 iMCD patients and 0 of 15 UCD patients studied. Further investigation is underway to identify the functional consequences, cell of origin, and frequency of these mutations in other patients through CDCN grants to Bob Ohgami, MD, PhD and Minji Byun, PhD.
Check out the full paper published in Blood Advances jounal here!
Want updates on the progress of all of our research updates? Check out our Research Pipeline for a full listing of CDCN research with a study description and funding status for each study.
4) Dr. Fajgenbaum speaking at a leading medical conference!
CDCN Co-Founder & Executive Director, David Fajgenbaum, MD, MBA, MSc, gave an address to hundreds of healthcare leaders at the Association of American Medical Colleges annual meeting about the CDCN’s unique approach to research and our life-saving progress for Castleman disease. We are so grateful for all of the incredible Castleman disease patient Warriors that have contributed samples, medical data, and donations to make this progress possible. Let’s build on our momentum and keep pushing things forward in 2019!
Watch Dr. Fajgenbaum’s speech here!
5) Results from a Cutting-Edge Technology to Study Castleman Disease Presented at ASH
This year’s American Society of Hematology (ASH) annual meeting had a record 8 presentations on research related to Unicentric and idiopathic multicentric Castleman disease!

Ruth-Anne Langan and members of the CSTL team at the ASH conference
The first research study that we’d like to highlight is led by Ruth-Anne Langan in Dr. David Fajgenbaum’s lab at the University of Pennsylvania and supported by funding from the CDCN and samples from many patients that generously donated flare and remission samples.
The researchers used a cutting-edge technology called “single cell RNA sequencing” to measure the gene expression and activity of thousands of genes in 10,000 circulating immune cells from patients in flare compared to gene expression of those cells to remission. They found several interesting changes in multiple immune cell populations including monocytes, plasma cells, and T cells, which could be playing critical roles in Castleman disease. These immune cell populations may also represent possible new targets for diagnostic tests or new treatment targets for Castleman disease patients. They are continuing to expand this study to include more patient samples thanks to patients generously donating samples and through caring individuals who are funding this work.
Want to help enable studies like this? Check out our donation page to help us fund more amazing progress!
6) Exciting Research Finding with Important Treatment Implications Presented @ ASH

Dustin Shilling, PhD
The next research study that we’d like to highlight is led by Dr. Dustin Shilling and Dale Kobrin in Dr. David Fajgenbaum’s lab at the University of Pennsylvania and is supported by funding from the CDCN, data from patients in the ACCELERATE Registry, and samples from many patients.
The researchers found significantly increased activity of a cellular pathway/communication line in lymph node tissue from idiopathic multicentric Castleman disease (iMCD) patients. The pathway, PI3K/Akt/mTOR, is important for cellular proliferation and production of cytokines that are elevated in iMCD. Most importantly, there are drugs that can inhibit this pathway. Further research into these drugs and expanding this study to include more samples is underway thanks to many patients and generous individuals who are funding this work.
Interested in donating your medical records to support research like this? Patients that want to contribute data to research can learn more about our ACCELERATE registry here!
Everyone can make a difference in the fight against Castleman Disease. Donate here!
7) New Sirolimus Clinical Trial!
Based on research done by Dr. David Fajgenbaum and the CDCN that found that a cellular communication line called mTOR is highly activatedin iMCD patients (and Dr. Fajgenbaum trying an mTOR inhibitor on himself in a Quest to cure himeself), a clinical trial will begin enrolling patients in 2019 to study the effectiveness of the mTOR inhibitor sirolimus in iMCD patients refractory to anti-IL-6 therapy.
This will be the first clinical trial in the US for idiopathic multicentric Castleman disease in over 5 years! Patients will be able to enroll at two sites: Univeristy of Pennslvania and the University of Arkanses for Medical Sciences. More info to come in 2019.
We are so grateful to the National Heart, Lung, and Blood Institute (NHLBI) and all of the generous donors and patients who have contributed data and samples to enable this groundbreaking research!
Learn more about David Fajgenbaum’s inspiring story in this 2017 NYTimes article here!
8) ACCELERATE Registry Progress
We have had 98 Castleman disease patients enroll into the ACCELERATE Registry this year! We are using data from these patients and hopefully hundreds more to identify and improve treatment and care for Castleman disease. In the words of David Fajgenbaum, CDCN Co-Founder and Castleman disease patient, “This is our best opportunity to do our parts, as patients, to help ourselves and other patients.”
All Castleman disease patients are encouraged to take just 15-20 minutes to enroll here.
Family members of deceased Castleman disease patients can also enroll deceased patients. We’ve had 8 courageous individuals enroll their deceased family members. Including these patients in the registry helps to better understand how to treat future patients and may save future lives.Please email info@castlemannetwork.org for more information about the registry.
If you haven’t registered yet, will you help us reach our goal of 100 in 2018?
9) Biobank Progress
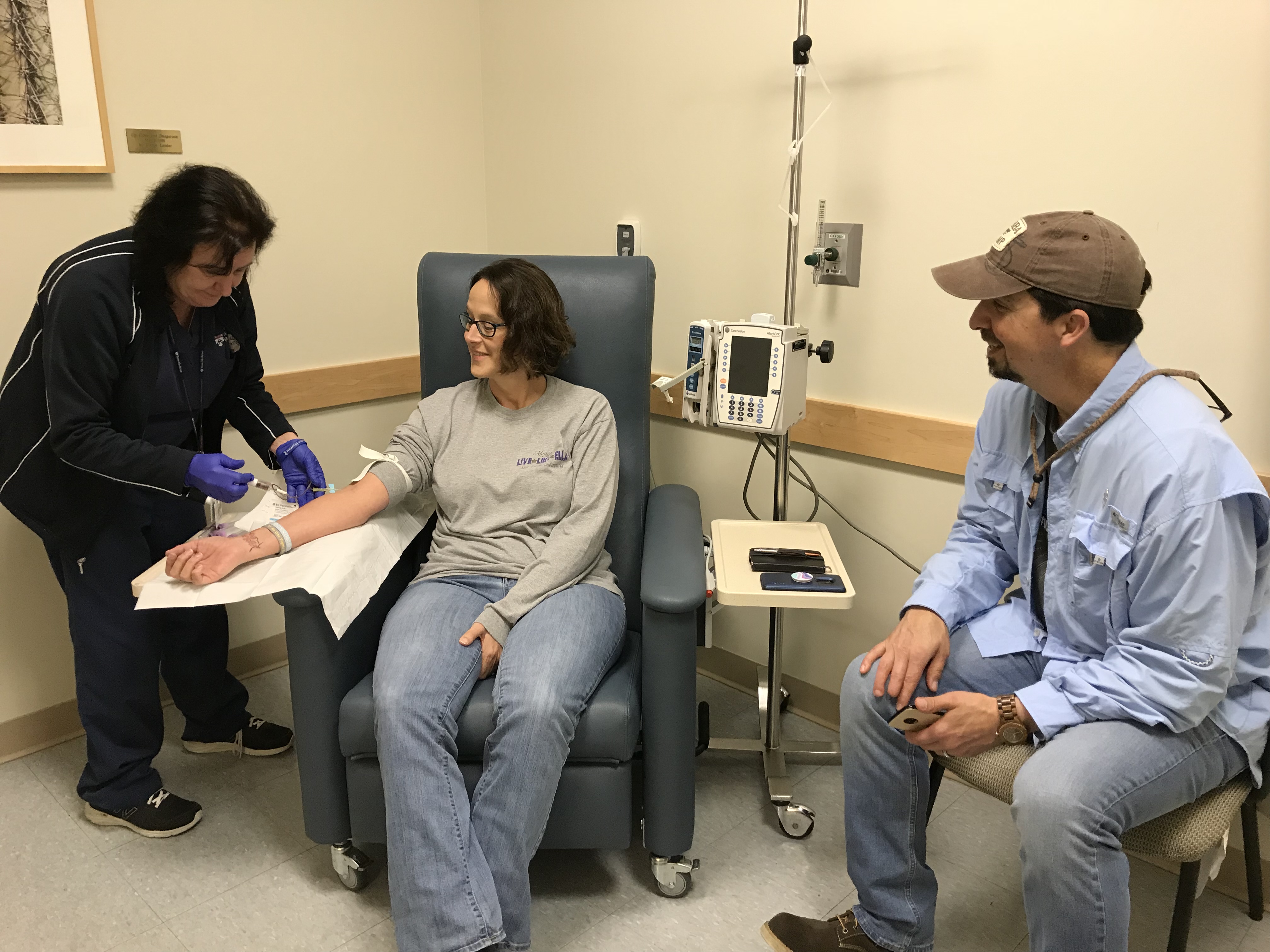 We have had over 60 patients contribute blood samples–and a few contribute excess lymph node tissue from a planned biopsy–to the new BioBank! Thank you so much to everyone who has contributed! None of the progress we’ve made would have been possible without these samples.
We have had over 60 patients contribute blood samples–and a few contribute excess lymph node tissue from a planned biopsy–to the new BioBank! Thank you so much to everyone who has contributed! None of the progress we’ve made would have been possible without these samples.
Now that the CDCN BioBank has been built, we need to get more blood samples from Castleman patients in the midst of flare/active disease and lymph node tissue when possible. Your blood sample or excess lymph node tissue may provide critical information to help better understand and treat this disease. In the words of David Fajgenbaum, CDCN Co-Founder and Castleman disease patient who has donated many samples himself, “The cure is within all of us.”
Please email CastleBank@uphs.upenn.edu or call 267-586-9977 if you’re experiencing Castleman disease symptoms or getting ready to have a lymph node removed. We handle all logistics, all costs, and make it donation very easy. You can express your interest in contributing future samples here.
10) SPEED-II Study Presented at ASH
Data from the SPEED-II study was published at ASH! This is a multi-year, 10-party collaborative study measuring >1300 molecules in over 350 samples, including samples from 100 iMCD patients and 100 related diseases. We partnered with Medidata, a top data science company, tofind new patient subgroups based on previously unknown proteomic signatures. We also found evidence of proteomic predictors of anti-interleukin-6 treatment response and gained novel insights into Castleman disease treatment and potential new drug targets.
Want updates on the progress of all of our research updates? Check our Research Pipeline for a full listing of CDCN research with a study description and funding status for each study.
Would you like to join in the fight against Castleman disease? Check out our donation page to help us fund future breakthroughs.
This has been an amazing year of progress, hard work, determination, and heart. Thank you so much being part of this journey as we keep fighting to cure Castleman disease together!


 We have had over 60 patients contribute blood samples–and a few contribute excess lymph node tissue from a planned biopsy–to the new BioBank! Thank you so much to everyone who has contributed! None of the progress we’ve made would have been possible without these samples.
We have had over 60 patients contribute blood samples–and a few contribute excess lymph node tissue from a planned biopsy–to the new BioBank! Thank you so much to everyone who has contributed! None of the progress we’ve made would have been possible without these samples.