Overview
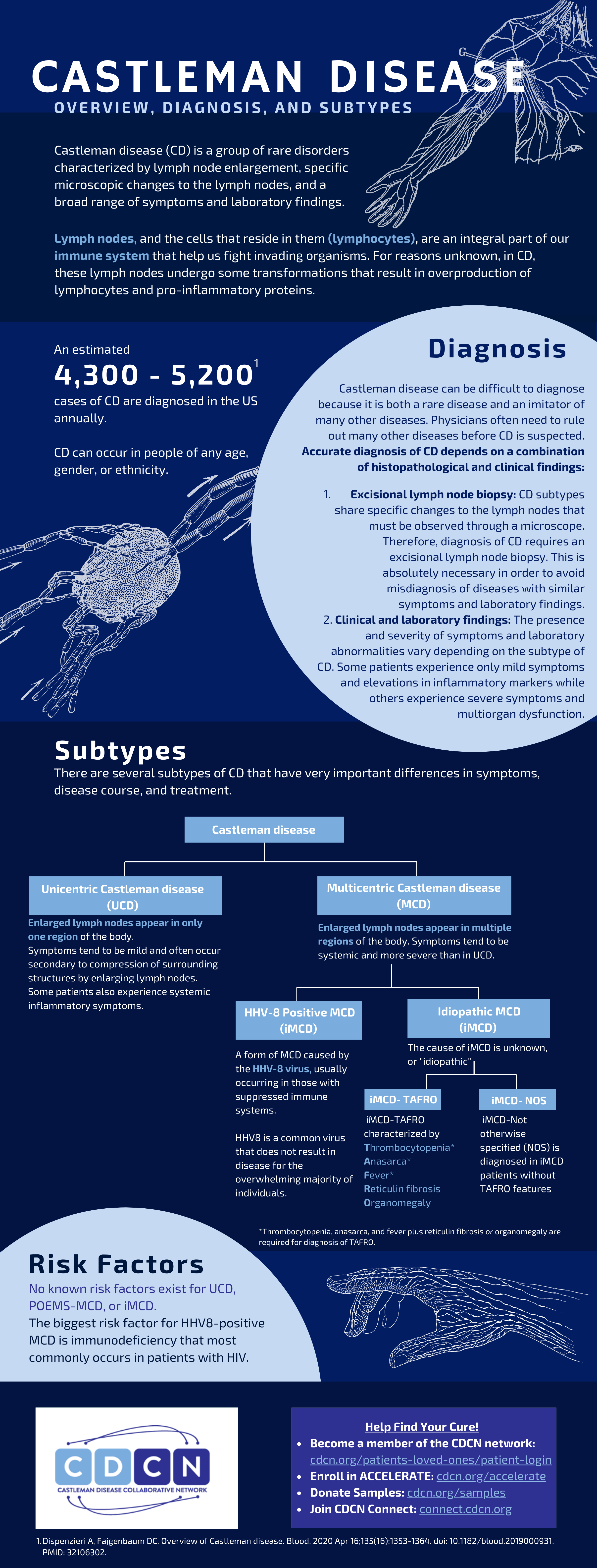
Castleman disease (CD) describes a group of rare disorders that involve enlarged lymph nodes with a similar lymph node appearance under the microscope and a broad range of inflammatory symptoms and laboratory abnormalities. There are four main subtypes of CD:
- Unicentric Castleman disease (UCD)
- POEMS-associated multicentric Castleman disease (POEMS-MCD)
- HHV-8-associated multicentric Castleman disease (HHV-8+MCD)
- HHV-8-negative/idiopathic multicentric Castleman disease (iMCD)
Disease overview
Castleman disease (CD) describes a group of rare disorders that involve enlarged lymph nodes and a broad range of inflammatory symptoms and laboratory abnormalities. The lymph nodes, and the cells that reside in them (lymphocytes and stromal cells), are an integral part of our immune system that help us fight invading organisms and cancer. In a healthy individual, the cells of the immune system become activated to fight off invading organisms or cancer and then return to a surveillance mode. In CD, the cells of the immune system become hyperactivated, overproduce cytokines and other inflammatory compounds, and fail to return to a surveillance mode.
Approximately 4,300-5,200 cases of CD are diagnosed in the US each year; CD can occur in people of any age, gender, or ethnicity. All forms of CD involve a constellation of microscopic abnormalities in the lymph node tissue that can be observed following a lymph node biopsy. Whether Castleman disease should be considered an autoimmune disease, cancer, or infectious disease is currently unknown. The symptoms, causes, and treatments vary greatly for each subtype of CD.
Subtypes
The main subtypes are Unicentric Castleman disease (UCD) and three subtypes of Multicentric Castleman disease (MCD). As the name suggests, the enlarged lymph nodes in UCD involve a single enlarged lymph node or multiple enlarged lymph nodes within a single region of the body that display microscopic features consistent with Castleman disease. UCD tends to have milder symptoms and rarely affects vital organs such as the liver, kidneys, and bone marrow. The three subtypes of MCD are characterized by lymph node enlargement in multiple regions of the body with characteristic microscopic features, flu-like symptoms, and organ dysfunction due to excessive cytokines or inflammatory proteins.
MCD is further sub-classified based on the underlying cause: POEMS-associated MCD, HHV-8-associated multicentric Castleman disease and idiopathic multicentric Castleman disease (iMCD).
POEMS-associated MCD involves a cancerous cell population found in patients with POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes) that can cause excessive cytokine production and MCD in a fraction of patients.
HHV-8 is a common virus that does not result in disease for the overwhelming majority of individuals. A small proportion of individuals infected with this virus, who are either HIV positive or have suppressed immune systems for another cause, develop HHV-8-associated MCD. In HHV-8+MCD, uncontrolled infection with HHV-8 causes the immune system to produce excessive cytokines that lead to MCD.
The most common subtype of MCD is idiopathic multicentric Castleman disease (iMCD). Though iMCD patients experience excessive cytokine production and a cytokine storm, the cause is unknown. There is no evidence of POEMS, HHV-8, or any other cancer or infectious disease in these patients. iMCD also has important differences in symptoms, disease course, and treatment from POEMS-associated MCD and HHV-8-associated MCD.
Furthermore, iMCD can be further sub-classified into three clinical subgroups:
- iMCD with TAFRO syndrome (iMCD-TAFRO) is characterized by acute episodes of Thrombocytopenia, Anasarca, Fever, Renal dysfunction or myelofibrosis, and Organomegaly
- iMCD with idiopathic plasmacytic lymphadenopathy (iMCD-IPL) is characterized by thrombocytosis, hypergammaglobulinemia, and a more chronic disease course
- iMCD, not otherwise specified (iMCD-NOS) is diagnosed in iMCD patients who do not have iMCD-TAFRO or iMCD-IPL.